An important breakthrough by Dr. Wang Changan of Tsinghua University and Dr. Li Ju of MIT promises to extend the lifespan of lithium-ion batteries that power today’s notebooks, mobile devices and other gadgets by a factor of four.
The unexpected discovery has the potential to allow for an all-week Apple Watch battery life while significantly extending run time between charges when using your iPhone, iPod touch, iPad or Mac notebook.
Remarkably, a team of forgetful researchers have figured out how to quadruple a li-ion battery’s lifespan literally by accident.
Basically, the researchers have managed to solved the problem of using aluminum for the anodes in the battery.
The traditional lithium-ion battery technology suffers from short lifespan stemming from the lithium compounds building up on graphite electrodes over time due to the anodes repeatedly expanding and contracting during the cycle of charging and discharging.
Lithium-ion usable lifespan
Over time, lithium compounds that build up on the electrodes break off, exposing the surface of the electrode. And when that happens, your battery’s capacity starts decreasing until it essentially becomes useless.
That’s why every battery inside your MacBook or iOS device has a limited number of full charge cycles. Your iPhone’s battery, for example, is designed to retain up to 80 percent of its original capacity at 500 complete charge cycles.
To find out your MacBook’s battery cycle count, read this tutorial.
Aluminum is the answer
Now, for a long time scientists have been pushing for the use of aluminum electrodes but couldn’t figure out how to pull it off as aluminum, as mentioned before, expands and contracts during the charging and discharging process.
As researchers focus on finding ways to stop the oxide coating from forming on the surface of aluminum nanoparticles when exposed to air, they thought about creating a new outer coating by soaking the nanoparticles in a sulfuric acid and titanium oxysuplphate mix.
This, they hoped, would dissolve the aluminum oxide and replace it with titanium oxide.
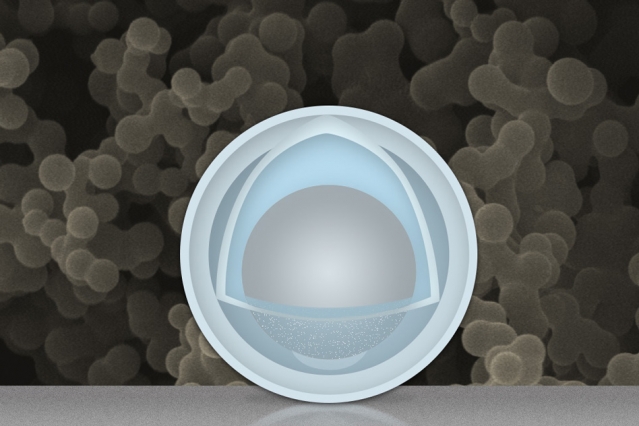
After forgetting to remove one batch of the nanoparticles from the soaking process for several hours, they made a startling discovery: the sulfuric acid and titanium oxysulfate mix dissolved some of the aluminum inside and left a 4nm outer shell of titanium hydroxide and an inner 30nm “yolk” of aluminum.
“The extra long soak meant the anodes did not expand and contract, in fact they created a battery that over 500 charge/discharge cycles retained up to four-times the capacity of the equivalent graphite anode batteries,” explains Geek.com.
Screenshot via apple.com/batteries.
“These batteries last considerably longer in terms of usable lifespan and, according to MIT, can hold up to three-times the energy.”
There was no word if this process can be easily applied to mass-scale manufacturing but it’s perfectly conceivable that much-improved lithium-ion batteries with at least four times longer lifespan could soon make their way into our iPhones, iPads and MacBooks.