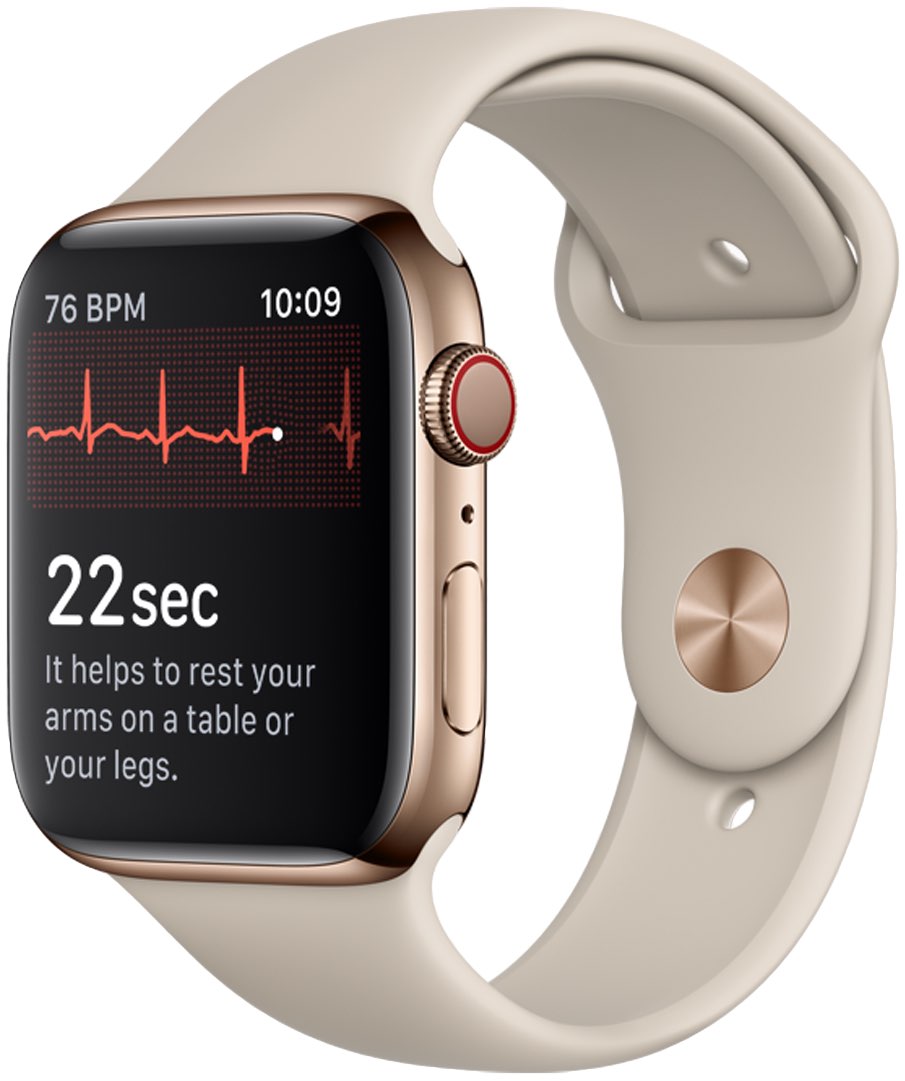
It could take years for a new electrocardiogram feature (ECG), which made its debut in the recently-released Apple Watch Series 4, to be officially approved in the United Kingdom.
The new electrical heart sensor inside the latest watches has been granted a De Novo classification by the FDA, a first of its kind. The sensor has the FDA’s clearance, not approval.
9to5Mac’s Ben Lovejoy reached out to UK’s Medicines and Healthcare products Regulatory Agency (MHRA) to ask what would be involved and how long the process would typically take.
“ECG devices for self-monitoring are classified as class 2a and the manufacturer will require a Notified body to carry out a conformity assessment,” they responded. “The most common assessment route is by audit of the full quality assurance system.”
While examining the official documentation and auditing Apple’s quality assurance system doesn’t sound that complicated, MHRA would also require an Apple clinical study measuring the effectiveness of the new sensor.
You may need to carry out a clinical investigation as part of the process to obtain a CE marking for your medical device. You must inform MHRA if you are planning to do this at least 60 days before starting your investigation providing some basic details about the investigational device, the intended population, the type of study and estimated application date.
Any additional requests could make the process longer.

Depending on the scale and the period over which the study is required to run, this could be an extremely lengthy approval process, the author adds.
“The last factor could be the most time consuming and could potentially add years onto the CE marking process,” the agency responded. The article speculates that Apple may have already obtained the necessary approval to conduct its existing heart study.
“Although the MHRA is the official UK body, because the UK is (for the moment) in the EU, Apple would have the option of obtaining permission from the equivalent agency in another European country,” reads the article.“The MHRA would not necessarily be aware of this.”

The ECG feature is arriving later this year via a watchOS software update.
Once it’s available in the United States, customers will be able to run an ECG scan of their heart at anytime simply by holding the finger against the Digital Crown, which will close the circuit between the electrodes in the Crown and on the back crystal. Measurements are automatically saved in the Health app and can be shared via a doctor as a PDF file.
Last week’s report by German publication Macerkopf.de alleged Apple has been working to bring support for the ECG app to Europe, without mentioning a possible timetable.